Gallery
 PM Modi visit USA
PM Modi visit USA Only the mirror in my washroom and phone gallery see the crazy me : Sara Khan
Only the mirror in my washroom and phone gallery see the crazy me : Sara Khan Karnataka rain fury: Photos of flooded streets, uprooted trees
Karnataka rain fury: Photos of flooded streets, uprooted trees Cannes 2022: Deepika Padukone stuns at the French Riviera in Sabyasachi outfit
Cannes 2022: Deepika Padukone stuns at the French Riviera in Sabyasachi outfit Ranbir Kapoor And Alia Bhatt's Wedding Pics - Sealed With A Kiss
Ranbir Kapoor And Alia Bhatt's Wedding Pics - Sealed With A Kiss Oscars 2022: Every Academy Award Winner
Oscars 2022: Every Academy Award Winner Shane Warne (1969-2022): Australian cricket legend's life in pictures
Shane Warne (1969-2022): Australian cricket legend's life in pictures Photos: What Russia's invasion of Ukraine looks like on the ground
Photos: What Russia's invasion of Ukraine looks like on the ground Lata Mangeshkar (1929-2022): A pictorial tribute to the 'Nightingale of India'
Lata Mangeshkar (1929-2022): A pictorial tribute to the 'Nightingale of India' PM Modi unveils 216-feet tall Statue of Equality in Hyderabad (PHOTOS)
PM Modi unveils 216-feet tall Statue of Equality in Hyderabad (PHOTOS)The Badminton Association of India (BAI) has announced a 14-member-strong India squad for
- Men’s Sr Hockey Nationals to be played in division-based format from April 4
- Mensik denies Djokovic 100th title in Miami final
- KIPG: Son of a vegetable vendor, Bihar’s Jhandu Kumar eyes Worlds, 2028 Paralympics
- Hardik Singh credits hard work and team unity for receiving HI Midfielder of the Year award
- Djokovic, Alcaraz land in same half of Miami draw
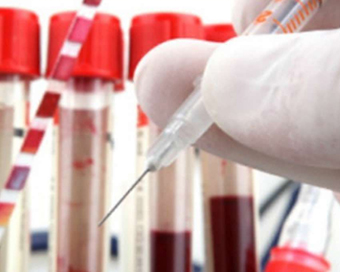
Trials of coronavirus vaccine begin in US Last Updated : 17 Mar 2020 10:49:51 AM IST 
US President Donald Trump (file photo) First trials have begun on a vaccine for the novel coronavirus ahead of the expected schedule, according to US President Donald Trump.
"This is one of the fastest vaccine development launches in history," he said at a news briefing on Monday.The private company, Moderna, which developed the vaccine, said that it had been injected to the first volunteer in Phase I of the trial. Forty-five volunteers were participating in the trial.Called mRNA-1273, it has to go through two more phases of trials to check for side effects and for efficacy before it can be deployed for mass vaccinations and the process can take at least a year.Moderna added that it was already preparing for Phase II trials of the vaccine, which could start in a few months.Trump said efforts to develop medications to treat the disease were also moving fast."We're also racing to develop antiviral therapies and other treatments. And we've had some promising results -- early results, but promising -- to reduce the severity and the duration of the symptoms," he said.Anthony Fauci, the administration's leading scientist dealing with the coronavirus crisis, said that he had expected it would take two to three months before the vaccine could be ready for trial, but it was ready in 65 days."I believe (this) is the record."The 45 healthy volunteers between the ages of 18 and 55 "will be followed for one year - both for safety and whether it induces the kind of response that we predict would be protective", he said.Fauci is the director of the National Institute of Allergy and Infectious Disease of the National Institute of Health, which is collaborating with Moderna in developing the vaccine.The trial is being conducted at the Kaiser Permanente Washington Health Research Institute in Seattle, Washington State where the first cluster of the disease in the US was found.In the first phase, Fauci said that the volunteers will be given "two injections: one at zero day -- first one; then 28 (after) days, there will be three separate doses: 25 milligrams, 100 milligrams, 250 milligrams".Moderna said it "continues to prepare for rapid acceleration of its manufacturing capabilities that could allow for the future manufacture of millions of doses should mRNA-1273 prove to be safe and effective".IANS New York For Latest Updates Please-
Join us on
Follow us on








172.31.16.186